Concepts from quantum mechanics lead to a powerful way of understanding the natural world.
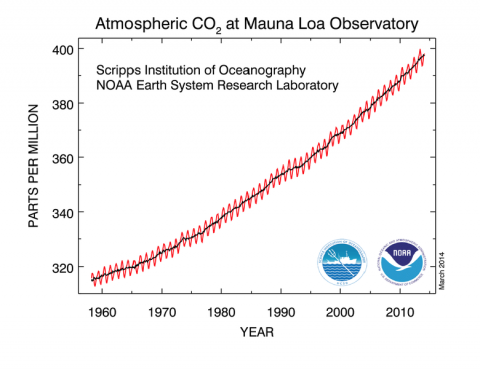
Our world is a raucous scene of atomic motion and molecular interaction. Although much of this commotion is too small for us to see, through chemistry we can make sense of the behavior of matter and explain the macroscopic world we experience through our senses.
Underlying this approach is a central idea in chemistry: the structure of an atom accounts for atomic properties. From this foundation, we can predict the physical and chemical behavior of atoms and molecules.
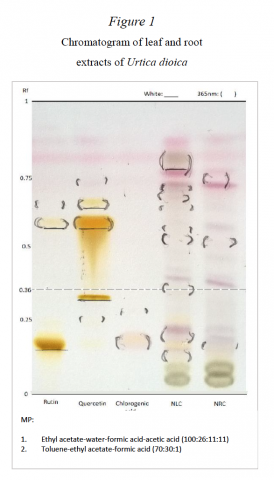
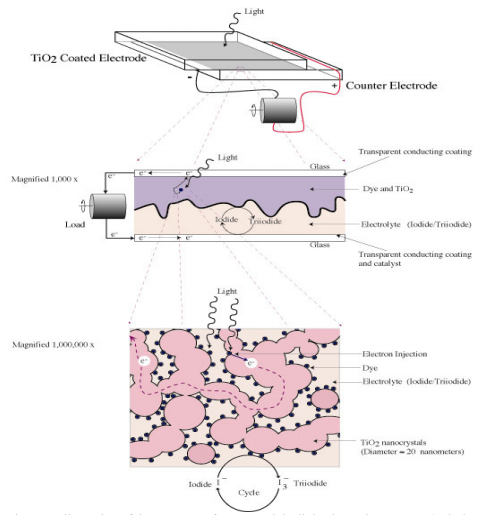
At Marlboro, students applied this core concept to explore topics that they found compelling, including subjects that range from biochemistry, environmental chemistry, and the history of chemistry to human medicine and advanced materials technology.